80% of Health Happens at Home: Why the Future of the Hospital Is Outside the Hospital?

Did you know that 80–90% of the most relevant health data occurs outside the hospital? Vitalera’s opportunity in the European Health Data Space
Today, 80–90% of the data needed to truly understand a person’s health status is generated outside the hospital: steps, sleep, home blood pressure, glucose, oxygen saturation, treatment adherence, daily symptoms… Yet most hospitals, insurers, and pharmaceutical companies only see a very partial snapshot of that reality.
At the same time, Europe is building the European Health Data Space (EHDS), the major European initiative where health data can be shared securely, interoperably, and with value for both care and research. In many documents and searches you may even find the term European Health Data Spcae, but they refer to the same thing: the European project aimed at making health data work together.
The real question is: who brings patient home data and hospital-at-home data into that European space, and who turns it into something truly useful for clinical care and pharmacovigilance? That’s where Vitalera has a unique opportunity.
Hospital at home: from a device in the living room to meaningful clinical data
For a CIO, medical director, or healthcare company, the challenge of hospital at home is not just delivering devices to patients, but:
- Ensuring those devices send data securely.
- Making the information arrive unified, not through ten separate platforms.
- Providing clinicians with data they can actually understand—not just isolated numbers.
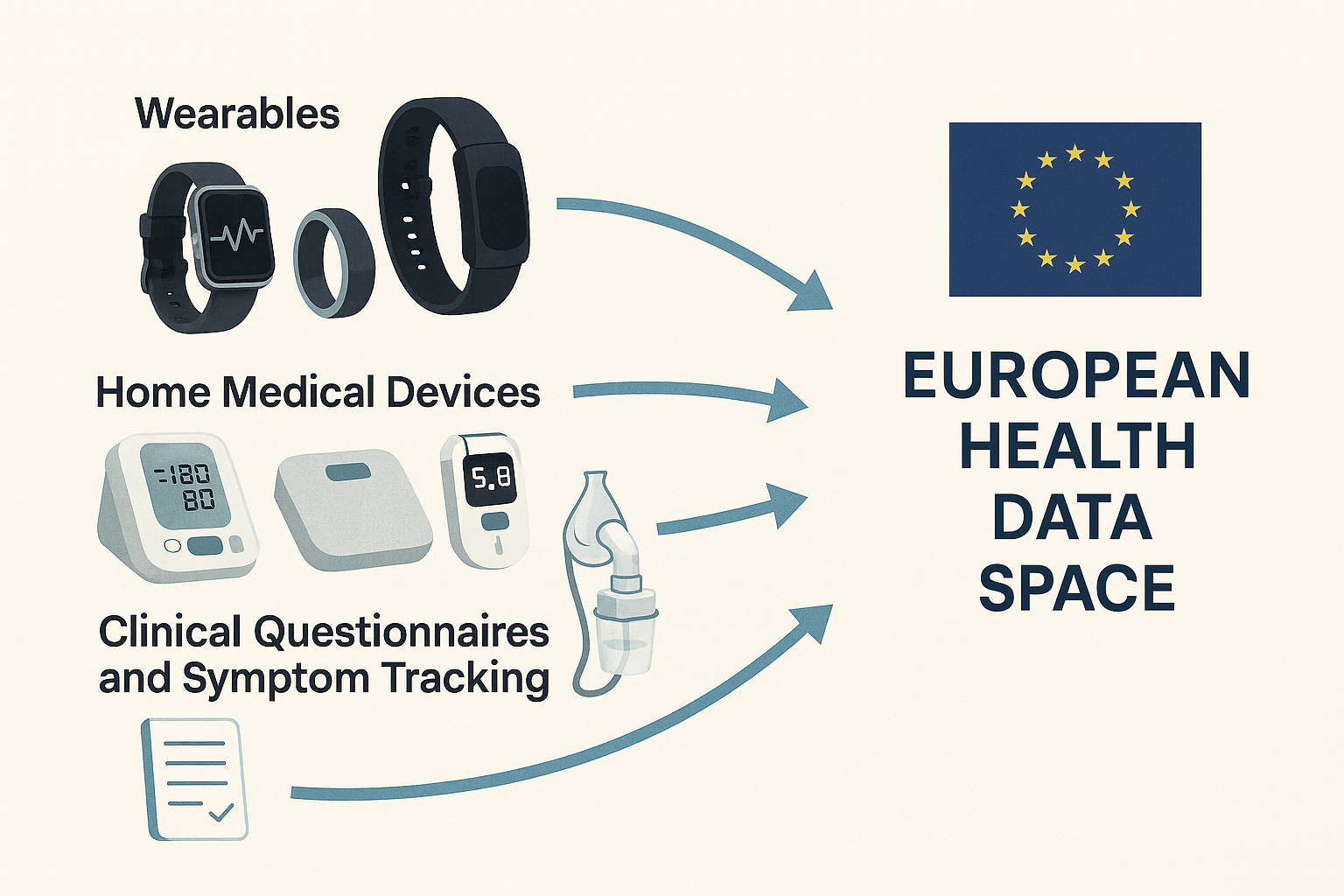
Vitalera acts as a single integration layer among:
- Wearables (watches, bands, rings, etc.)
- Home medical devices (blood pressure monitors, scales, glucometers, pulse oximeters, nebulizers, respiratory devices, etc.)
- Clinical questionnaires and symptom tracking through the patient app.
Instead of hospitals having to integrate with each vendor separately, Vitalera provides one API for medical devices—a single technical integration enabling dozens of devices. For the clinician, this means clear, continuous visibility of the patient at home; for IT departments, less complexity and more control.
The opportunity in pharmacovigilance: real-world data, not only hospital data
Traditional pharmacovigilance relies on:
- Spontaneous reports of adverse events.
- Manual chart reviews.
- Data limited to what happens in appointments or inpatient care.
But many side effects, adherence issues, and early warning signs appear in daily life, not in the hospital. This is where combining wearables with home medical devices changes everything:
- We can detect changes in heart rate or sleep patterns after starting a treatment.
- We can identify drops in adherence before a major deterioration occurs.
- We can cross-reference patient-reported symptoms with objective evolution data.
Vitalera transforms all of this into structured and anonymizable data, ready to feed pharmacovigilance systems and Real World Data / Real World Evidence projects aligned with the future EHDS. For pharma or research units, this means moving from small, partial samples to continuous, high-quality real-world data.
Four reasons to choose vitalera as your EHDS partner:
1. Connecting hospital at home with the “real” hospital
Vitalera ensures that hospital-at-home programs stop functioning as isolated projects and instead become a natural extension of the hospital:
- Device and wearable data are sent automatically.
- Integrated into simple clinical dashboards with configurable alerts.
- Ready to be pushed into the EHR or the hospital’s data lake.
For clinicians, it means seeing the patient even when they’re in their living room.
For management, it means fewer readmissions, better patient experience, and increased bed capacity without compromising safety.
2. One single technical infrastructure for many devices
From an IT perspective, a common fear is ending up managing:
- Five different vendor portals.
- Eight different APIs.
- Endless updates and integrations.
With Vitalera, everything is simplified into:
- A single integration API for all certified devices.
- A unified data model that maps easily to internal systems.
- One central point for monitoring, auditing, and security.
In short: technical simplicity and scalability, aligned with Europe’s interoperability standards.
3. Pharmacovigilance and Real World Data ready for research
For clinical research departments, trial units, or pharma companies, Vitalera provides:
- Continuous real-world patient data, not just follow-up visit data.
- Early safety, tolerability, and adherence signals.
- The ability to design decentralized or hybrid clinical trials with in-home measurements.
These data can:
- Be anonymized and formatted for analytic platforms.
- Be shared—with proper consent—with consortia or projects connected to the European Health Data Space.
Pharmacovigilance becomes proactive and evidence-driven, not just reactive.
4. Regulatory compliance and patient trust by design
None of this works without patient trust. Vitalera includes:
- Clear, informed, and reversible consent flows.
- Tracking of which data is used, by whom, and for what purpose.
- Strong pseudonymization and anonymization aligned with GDPR.
This allows hospitals, health companies, and pharma to:
- Unlock the value of home-generated patient data.
- Do so within a framework fully compatible with the European Health Data Space, ready for audits and international collaboration.
In summary
If most relevant health data is generated outside the hospital, and Europe is building the European Health Data Space to make better use of this information, the opportunity is clear:
- For hospital at home: to see and care for patients continuously, not just when they walk into the hospital.
- For pharmacovigilance: to detect earlier, understand better, and act faster using real-world data.
Vitalera is uniquely positioned as the layer that connects devices, patients, and healthcare systems—turning scattered home data into useful, interoperable, and secure information ready for the ecosystem of the European Health Data Space / European Health Data Spcae.
For CIOs, healthcare companies, clinicians, and pharmaceutical organizations, the proposition is simple:
less technical complexity, more relevant data, and greater clinical and research value from the patient’s home.
Related content
CPT Code 99453 - Remote Patient Monitoring
Discover the essentials of CPT Code 99453 and how it supports reimbursement for Remote Patient Monitoring (RPM). Our mission is to help you maximize compliance and secure reimbursement for remote care services. Learn about CPT Code 99453's role in RPM setup, patient education, and device connectivity. Access our guide on launching an RPM program, and explore how to leverage CPT codes for seamless RPM billing and Medicare compliance.

5 benefits of using AI in healthcare with connected health APIs
Telemonitoring with quality data represents a substantial change in healthcare, improving chronic disease management and patient outcomes. Technologies like wearables and telemedicine enable real-time monitoring, early detection, and personalized care, reducing hospital readmissions and urgent visits. Vitalera's interoperable platform integrates with over 500 devices, leveraging AI for enhanced patient monitoring, early disease detection, and efficient healthcare delivery.

vitalera and Maastricht University Medical Center Partner to Remote Monitor Post-DIEP Procedure Recovery
Vitalera, a leading medical platform in remote patient monitoring, partners with Maastricht University Medical Center (MUMC) to revolutionize post-DIEP recovery. Through AI-driven analytics, wearable tracking, and real-time monitoring, Vitalera enhances patient outcomes, reduces hospital readmissions, and sets a new standard in post-operative care.










